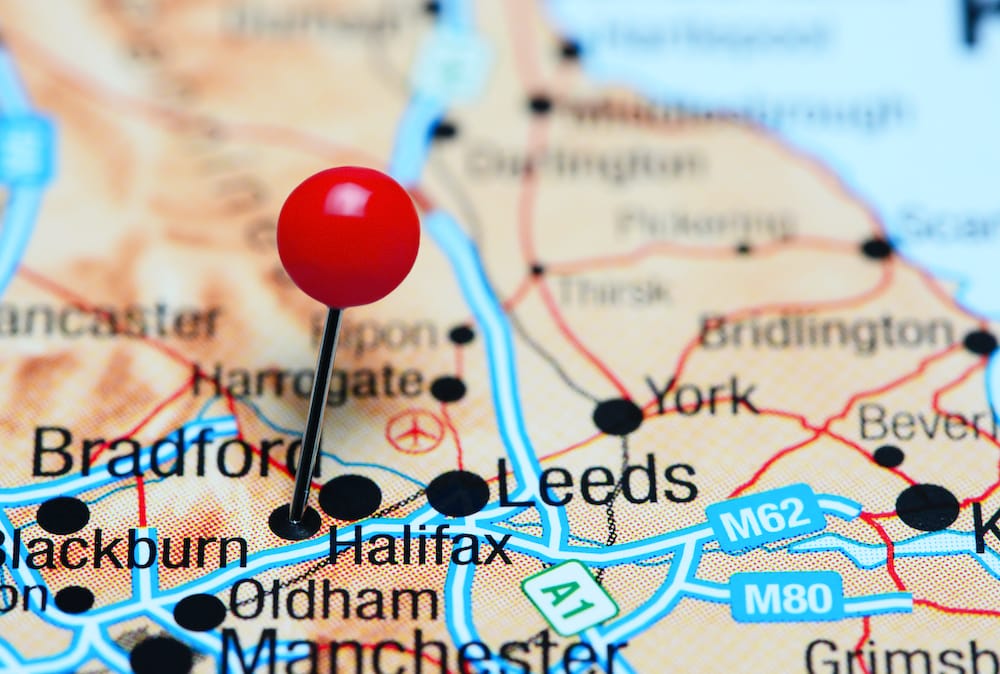
Industry
Big plans afoot at Halifax HQ
-

 News6 months ago
News6 months agoEurope’s first legal cannabis dispensaries to open under sixth Swiss pilot
-

 Science5 months ago
Science5 months agoMedical cannabis doesn’t impair cognitive function – study
-

 Industry6 months ago
Industry6 months agoFirst Jersey-grown medicinal cannabis product to hit UK market
-

 News6 months ago
News6 months agoFive years of medical cannabis, five NHS prescriptions – how did we get here?
-

 News5 months ago
News5 months agoJapan takes major step towards cannabis reform
-

 Health5 months ago
Health5 months agoUK data links medical cannabis to improvements in ADHD patients
-

 News5 months ago
News5 months agoMPs call for children with epilepsy to get medical cannabis on NHS
-

 Health3 months ago
Health3 months agoUK researchers publish first findings on cannabis and Long COVID