A new report explores the progression of cannabis research over the last decade, as well as the barriers holding back further developments in the pharmaceutical space.
We take a look at some of the key takeaways.
The report, published today by Prohibition Partners and Cannabiscientia, gives an in-depth account of all activity involving cannabinoids in the global pharmaceutical landscape over the last 13 years.
It includes a breakdown of over 400 clinical trials, registered patents in the field, as well as analysis of research funding, mergers and acquisitions and market sizing forecasts.
Analysts also explore the main barriers that are preventing further research and, ultimately, the development of new cannabis-related treatments.
Here are some of the key findings:
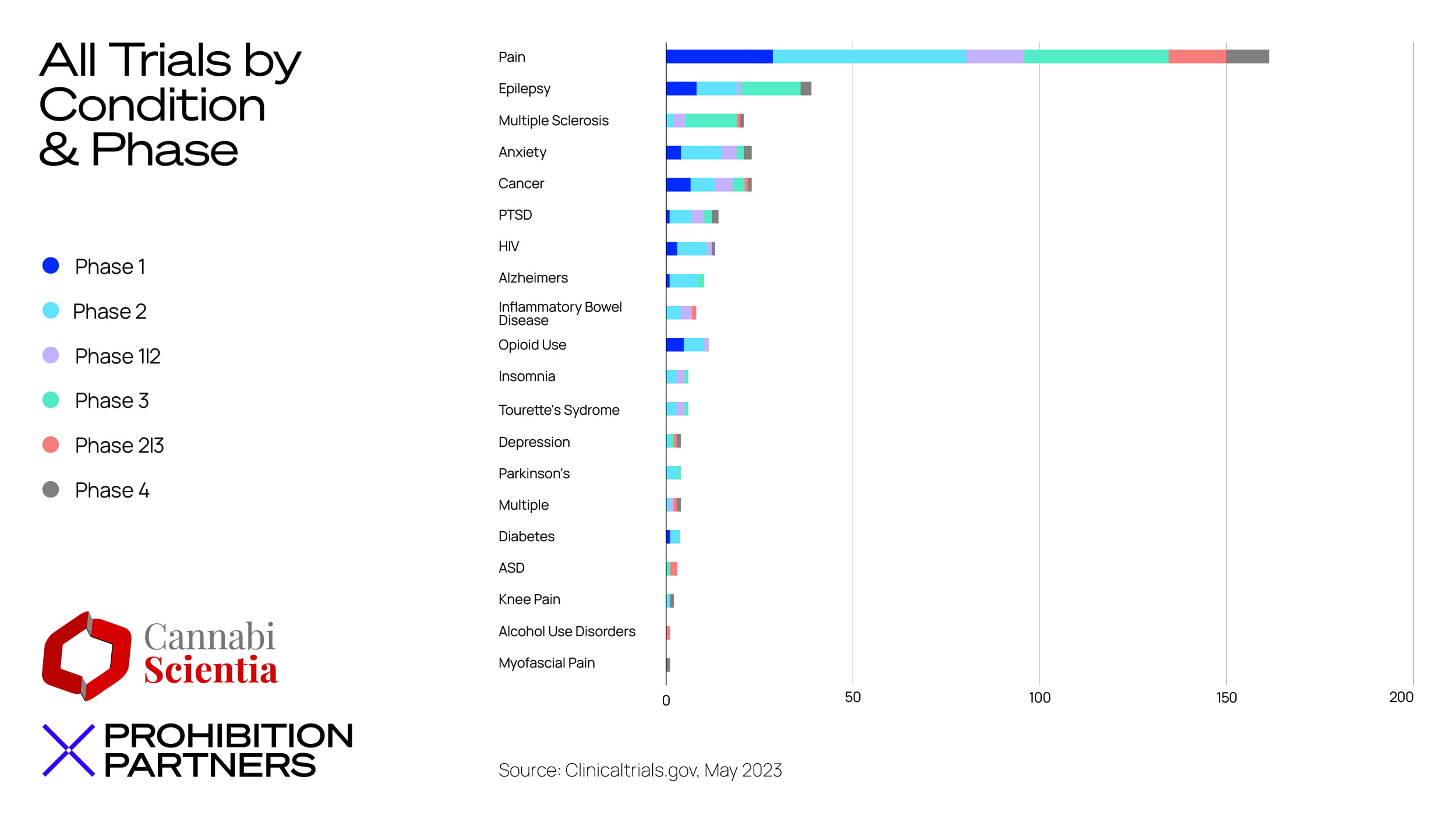
– Pain, across a number of different diagnoses, was the most common indication treated in cannabis-related clinical trials, with findings consistently showing ‘promising potential’ in this area.
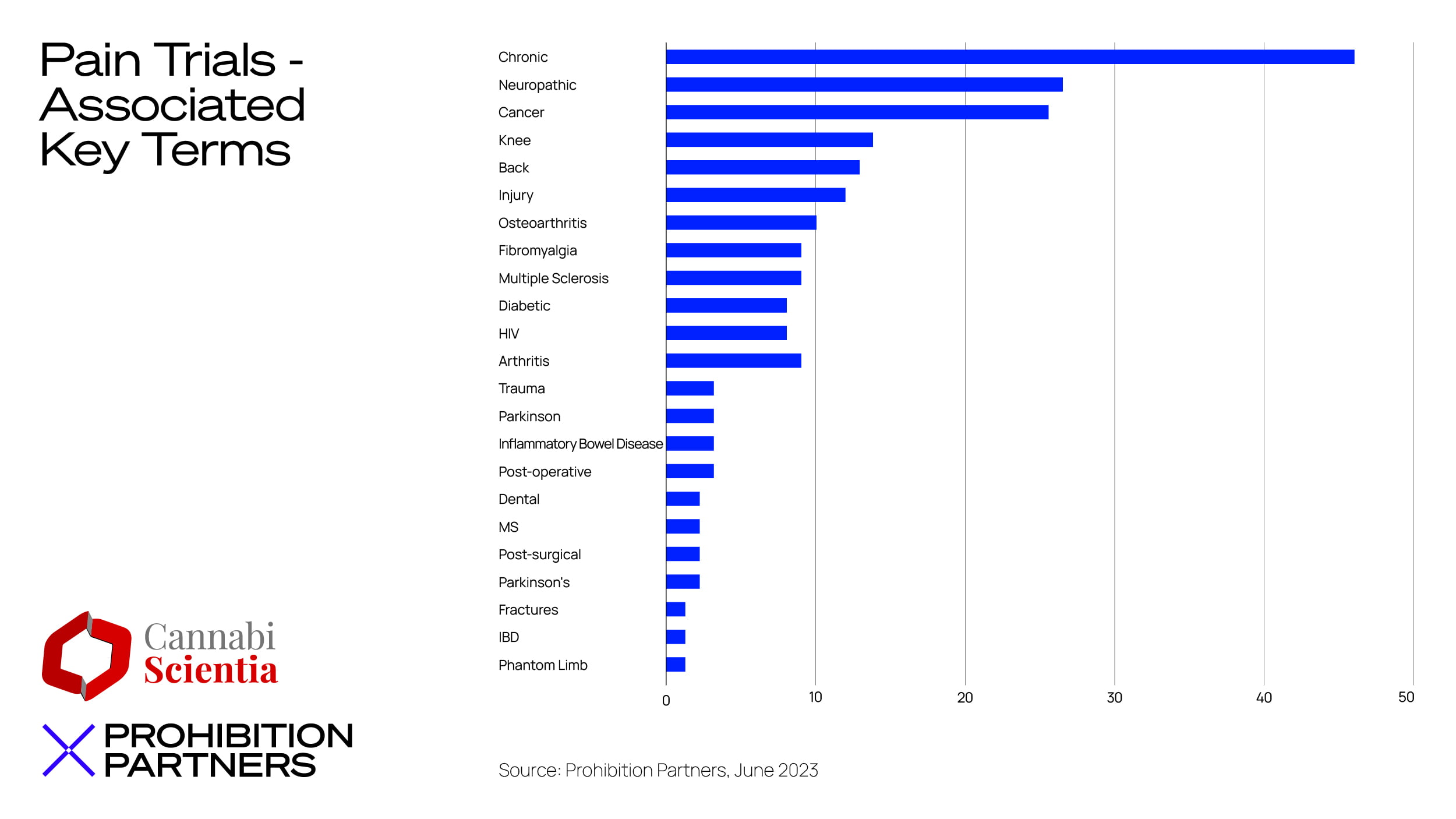
– There has been a notable increase in clinical trials examining the potential benefits of cannabinoids for various psychiatric disorders, specifically, in managing common mental health conditions, such as anxiety and depression.
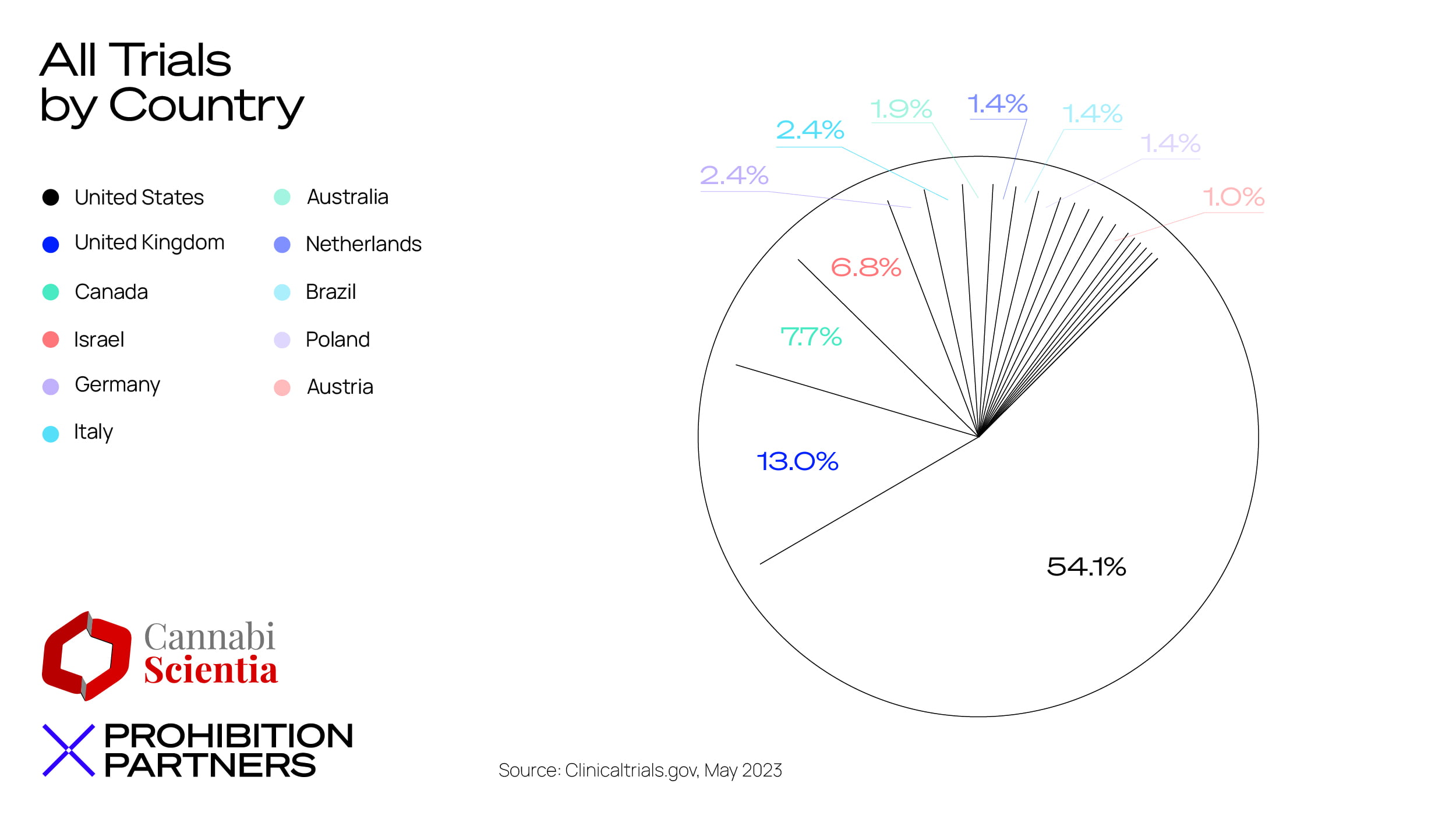
– The UK saw the second highest number of trials registered via clinicaltrials.gov, making up 13% of all trials during the last 13 years. This followed the US, where the majority (54%) of trials were registered.
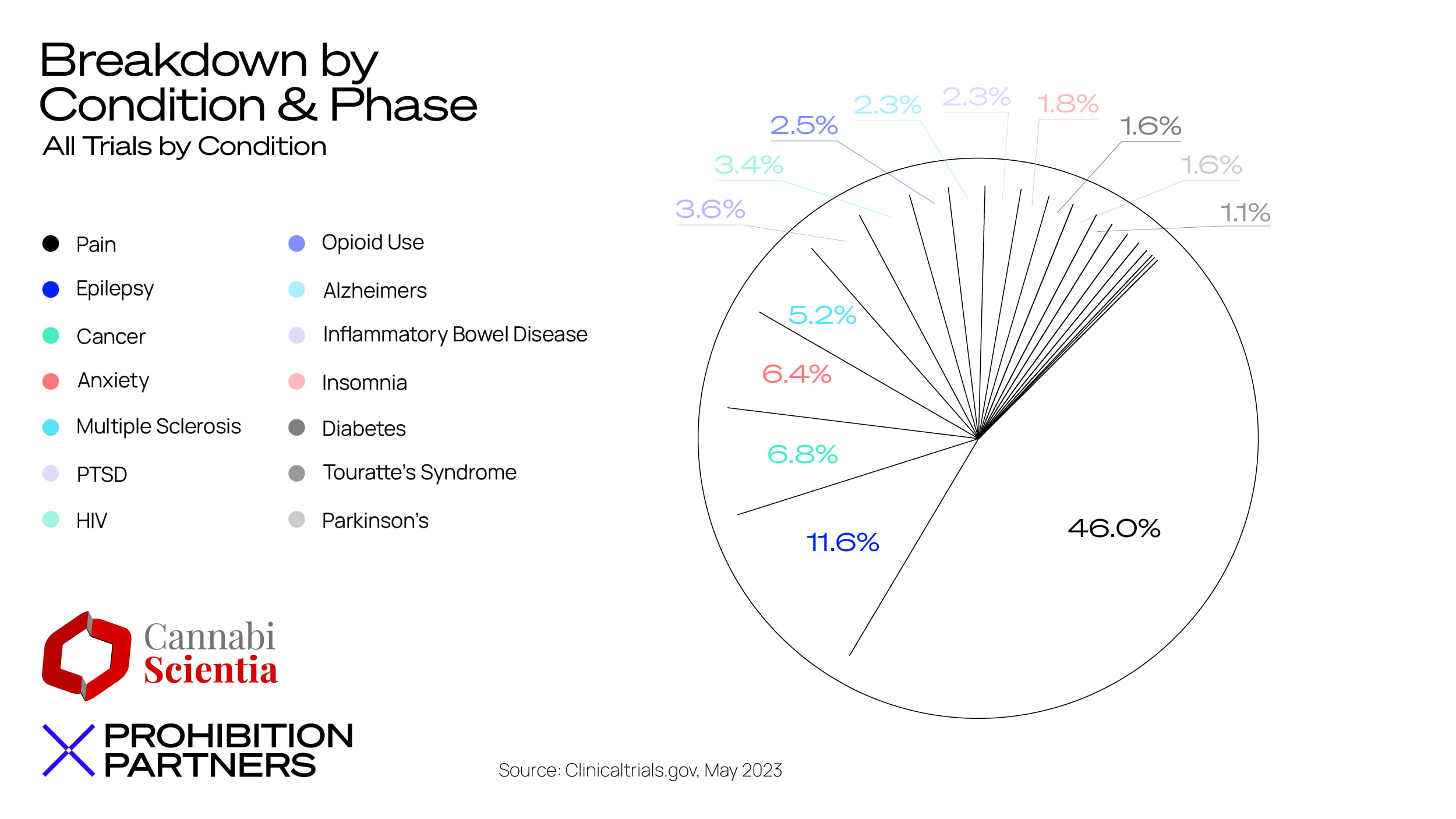
– CBD in isolation from other cannabinoids is generating a significant amount of activity as a treatment in clinical trials – approximately 50% of all trials beginning in 2022 onwards included only CBD. However, over the past 13 years there has been a larger focus on treatments involving multiple cannabinoids (primarily THC and CBD).
– CBD and THC are still seeing the majority of patent activity versus other cannabinoids, but activity is also focused on cannabinoids and compounds that are closely related for example, CBDV, THCV and CBDA.
– The conditions which cropped up most often in registered patents include epilepsy, cancer and associated conditions, seizures, and autism spectrum disorder.
– GW Pharmaceuticals (now owned by Jazz Pharma) and its research subsidiary, GW Research Ltd, continues to dominate in the sector, having sponsored more clinical trials in the past 10 years than the next five leading sponsors combined, and with more patents any other company.
– Epidiolex, produced by Jazz, makes up the majority of global pharmaceutical cannabis sales, with an estimated market share of around 76% projected for 2023.
– Global sales within the pharmaceutical cannabis industry are estimated to reach approximately US$1.11 billion in 2023, with projected growth to US$1.37 billion by 2027 (based primarily on the sales of three licensed cannabinoid treatments: Epidiolex, dronabinol, and Sativex).
– Clinical trials featuring Sativex and Epidiolex also dominate completed phase 3 trials.
– While there are no obvious patterns for success in cannabis-related clinical trials, the most common reason for trial discontinuation is a lack of funding, according to the report. A phase 3 trial is estimated to cost between $11.5m – $52.9 to complete.
– The main barriers holding back research and development of new cannabinoid-based treatments include: the lack of standardisation of cannabis-based medicines; the challenges in designing placebo-controlled trials on cannabinoids and the difficulties in establishing intellectual property (IP) around cannabinoid treatments [it is difficult to patent treatment with medicine which comes directly from plant material].
The Pharmaceutical Cannabis Report: 3rd Edition is now available to purchase via the Prohibition Partners website. Alongside the report, additional data and insight packages are available, including:
- Global Clinical Trials Database: A comprehensive list of 440 clinical trials involving cannabis and cannabinoids taking place since 2010.
- Patent Portfolio: A comprehensive review of the patent portfolios of Jazz Pharmaceuticals Ltd. (split into those held by GW Research Ltd., and those held by GW Pharmaceuticals Ltd.), and of six other leading companies involved in patenting activity for the medical application of cannabis and cannabinoids
Home » Industry » The state of pharmaceutical cannabis – 12 key takeaways

 News6 months ago
News6 months ago
 Science5 months ago
Science5 months ago
 Industry6 months ago
Industry6 months ago
 News6 months ago
News6 months ago
 News5 months ago
News5 months ago
 Health5 months ago
Health5 months ago
 News5 months ago
News5 months ago
 Health3 months ago
Health3 months ago
















