More than 450 cannabinoid clinical trials have taken place or are currently underway, Miguel Fagundes, chief technical officer at Eurocan explains everything you need to know about these studies.
Sponsored
The rapid growth of cannabis-based medicines is creating significant disruption within the global pharmaceutical industry and changing the way the pharmaceutical industry approaches cannabis, as pharmaceutical cannabis products are becoming genuine alternatives to traditional pharmaceuticals for certain treatments, conditions and diseases.
Clinical trials and academic studies are increasing around the world constantly thereby increa
sing our understanding of the properties of cannabis plant.
Where?
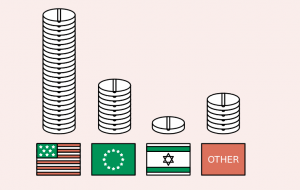
Cannabinoid related trials and studies are taking place all around the world. Most ongoing or upcoming clinical trials are taking place in the US where the large population pool, a developed network of experienced investigators, a supportive regulatory framework, and a vast market of drug consumption all combine to support expansion in clinical trials.
The UK and the Netherlands are currently responsible for around 71 percent of the active cannabinoids clinical trials in Europe, a reflection of the fact these countries were among the first movers in easing the regulatory and legal barriers to commercial research and clinical trials. Elsewhere, Israel also has a significant role in cannabis related research with involvement in over 20 active/completed clinical trials.
What?
Over 250 clinical trials have already been completed and there are more than 200 active clinical trials in process around the world.*
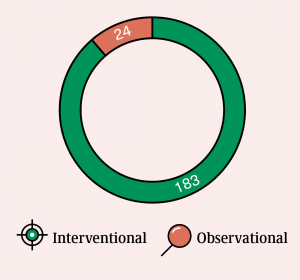
Interventional Studies:
This type of study dictates that the participants are assigned into groups that receive one or more intervention/treatment (or no intervention) so the effects on biomedical or
health-related outcomes can be evaluated by researchers.
Observational Studies:
Participants belong to study groups and are assessed for biomedical or health outcomes.
The investigator does not assign participants to a specific intervention/treatment.
The majority of on-going cannabinoids-related studies are “interventional” in nature (over 90 percent), in line with regulators’ preference that evidence be obtained in a controlled manner, with participant groups monitored in a controlled environment. Recent information shows that a large number of trials are actively recruiting, in addition to those which have already been completed. This indicates the scientific and medical commitment to continue to move forward with the clinical research to prove clinical relevance and therapeutic potential of these cannabinoid molecules.
Study design
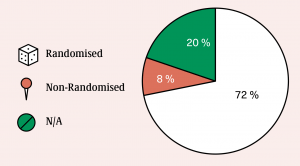
Clinical trials in relation to cannabinoids are generally (72 percent) designed so as to ensure a random allocation of intervention/treatment among the trial participants. A typical study design will include placebo-controlled, randomised, single-ascending dose and multiple- ascending dose studies. This structured and standardised approach produces effective and reliable scientific evidence on the effectiveness and safety of cannabinoids, without bias.
Purpose of trials
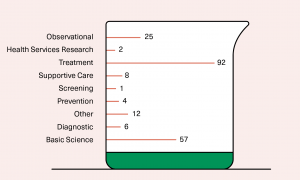
The main purpose of current clinical trials into cannabinoids is to search for new treatments and to collect scientific data. The scientific data obtained through clinical trials explains how cannabinoids interact with receptors within the human body. Clinical trials also assist in understanding the physiological responses by participants during trials.
How?
The main sources of funding clinical trials and studies are academic research institutions, private enterprises and national governments. Each of these are key players in the process of adoption and access to cannabinoids for end-users. Most studies to date have been funded by academic institutions, but in recent times there has been a significant increase in the number of joint ventures and research projects with private enterprises. A significant proportion (roughly 20%) of studies are entirely privately funded, with only a small proportion being funded by governments.
Conclusion
More than 450 clinical trials studying cannabinoids as potential drugs with clear therapeutic application have been completed or are in progress. Minor cannabinoids are also gaining attention within the research community and this will further broaden our knowledge of the full properties of the cannabis plant.
It is clear that this level of interest and the increasing knowledge base surrounding the endocannabinoid system, cannabinoids and cannabis-based medicines have the potential to change the way we medicate in future years. The commitment to scientifically robust evidence and data driven product development within the cannabis industry will only benefit end users and assist in the growth of a regulated market to develop new and innovative products.
Read more: The clinical trials roadmap: Everything you need to know about the trialing of new medicines
* Publicly availing information regarding clinical trials is available at clincaltrials.gov, clinicaltrialsregister.eu and health-products.canada.ca.
References: Clinicaltrialsregister.eu / Clinicaltrials.gov / WHO / INFARMED / NICE
info@euro-can.eu / www.euro-can.eu

 Science5 months ago
Science5 months ago
 News6 months ago
News6 months ago
 News6 months ago
News6 months ago
 Health5 months ago
Health5 months ago
 Health4 months ago
Health4 months ago
 Science6 months ago
Science6 months ago
 Cannabis explained6 months ago
Cannabis explained6 months ago
 Science6 months ago
Science6 months ago
















